Ferric Pyrophosphate Citrate (FPC) Program
Rockwell Medical’s proprietary parenteral iron product, Triferic® (ferric pyrophosphate citrate (“FPC”)) is indicated to maintain hemoglobin in adult patients with hemodialysis-dependent chronic kidney disease.
Rockwell Medical began commercializing Triferic (dialysate) and Triferic AVNU (intravenous) in the United States in the second half of 2019 and in early 2021, respectively. Triferic was launched into a very competitive marketplace with well-entrenched products and a lack of consensus regarding unmet medical needs for dialysis patients with anemia. Due to its limited market adoption, unfavorable reimbursement, and absence of interest from other companies to license or acquire Triferic despite Rockwell’s significant effort to partner the program, Rockwell Medical discontinued its New Drug Applications (“NDAs”) for Triferic and Triferic AVNU in the United States in the fourth quarter of 2022.
International Partnerships
While Rockwell has discontinued commercialization of Triferic in the United States, the Company has established international partnerships with companies seeking to develop and commercialize Triferic outside the United States and is working closely with these international partners to develop and commercialize Triferic in their respective regions.
South Korea
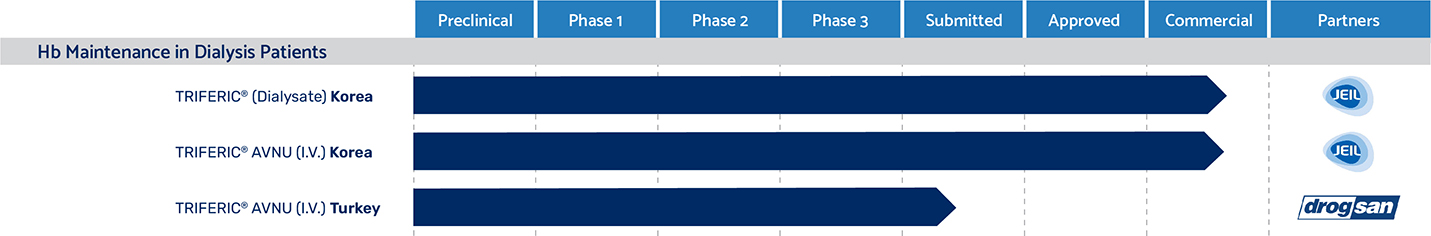
Triferic and Triferic AVNU have been approved in South Korea, and our partner, Jeil Pharmaceutical, launched Triferic in South Korea during the third quarter of 2022.
Turkey
In April 2023, Drogsan submitted a Marketing Authorization application and GMP application for Triferic AVNU to the Turkish Medicines and Medical Devices Agency ("TMMDA"), for which Drogsan received priority status and high priority status, respectively. Taking into consideration that Drogsan was granted an accelerated review for Triferic AVNU with the Turkish regulatory authority, Rockwell anticipates approval for Triferic AVNU in Turkey in 2024. Drogsan is responsible for all regulatory approval and commercialization activities.